Unstable life, unstable work, unstable relationship…why oh why?
Don’t know why either.
Maybe we could learn something at microscopic viewpoint.
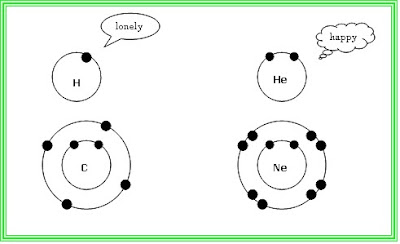
As mentioned before, naturally electrons are unstable, except for inert gases, you’ll learn later why.
But first let me introduce you first to a very important tool – The Periodic Table!
(this is only a part of Periodic Table since I just took a print screen, if you want the complete version get it from www.ptable.com )
The number of electrons an element has depends on the atomic number. It is the number that is usually placed as superscript.
So Hydrogen H has one valence electron, Helium He has 2 and so on.
The first energy level must be filled with 2 electrons to be stable, then the following energy levels must have 8 electrons.
So basically only those inert gases (on the most right side column) are stable and the rest of the elements seek their destined partners.